

By contrast, the peripheral polypeptide cytochrome c readily dissociates from the cristal membrane, making it easy to purify. The very presence of the hydrophobic alpha-helical domains in trans-membrane proteins makes them difficult if not impossible to isolate from membranes in a biologically active form. Integral membrane proteins that do not span the membrane also have a hydrophobic helical domain that anchors them in the membrane, while their hydrophilic domains typically interact with intracellular or extracellular molecules to e.g., hold cells in place give cells and tissues their structure, etc.

Because of these hydrophilic interactions, such proteins can create pores for the transport of polar molecules and ions we will see some of these proteins later. Proteins that span membranes multiple times may include amino acids with charged, polar side chains, provided that these side chains interact between helices so that they are shielded from the fatty acid environment in the membrane. Glycophorin A monomers pair to form dimers in the plasma membrane. One glycophorin A polypeptide with its hydrophobic trans-membrane alpha helix is cartooned below. As an example, consider the amino acids in the alpha-helical domain of the red blood cell protein glycophorin A, a membrane protein that prevents red blood cells from aggregating, or clumping in the circulation. The alpha helical domains that anchor proteins in membranes are mostly non-polar and hydrophobic themselves.
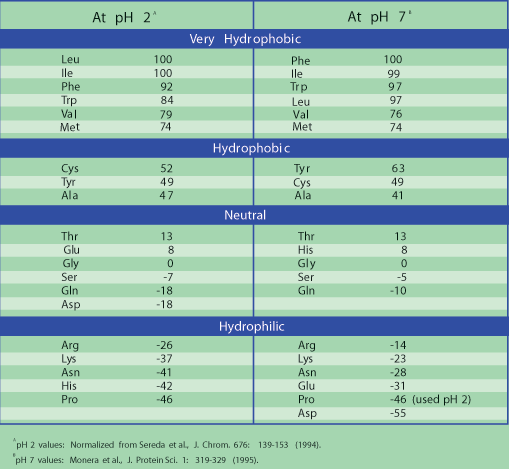
N-terminal end of a plasma membrane polypeptide always ends up exposed to the outside of the cell. Transmembrane proteins can in fact cross a membrane more than once, which also determines the location of its N- and C-termini. How a transmembrane protein inserts into the membrane during synthesis dictates the locations of its N- and C-terminus. The protein on the left crosses the membrane once, while the one on the right crosses the membrane three times. Two trans-membrane proteins are cartooned below. Hydrophilic domains tend to have more tertiary structure with hydrophilic surfaces, and so face the aqueous cytosol and cell exterior. The hydrophobic domain of integral membrane proteins consists of one or more alphahelical regions that interact with the hydrophobic interior of the membranes.


 0 kommentar(er)
0 kommentar(er)
